Cases, Deaths, and Vaccination Rates
Hepatitis B (hep B), according to the CDC, is “a contagious virus that is transmitted through blood, blood products, and other body fluids (such as semen)… Symptoms include a sudden fever, tiredness, loss of appetite, nausea, vomiting, stomach pain, dark urine, joint pain, and yellowing of the skin and eyes (jaundice).”

Source: Sanofi Pasteur, “Hepatitis B Virus (HBV),” flickr.com, Aug. 7, 2013
In 1965, Baruch Blumberg, an American doctor who won the Nobel Prize in Medicine (1976) for his work on hepatitis B, matched a protein found in an Australian aborigine’s blood with an antibody found in an American hemophiliac. First called the “Australian antigen,” it was discovered to be the hepatitis B virus and provided a source for the vaccine created in 1969. Because the virus could not be recreated in a lab, the first vaccine was a heat-treated form of the virus.
In 1981, the FDA approved Heptavax-B, a vaccine created by Maurice Hilleman. Because Heptavax-B used human serum and the fear of HIV infection was high, a new recombinant DNA vaccine, Recombivax HB, was licensed on June 23, 1986 that did not use human serum. As of July 2014, two hepatitis B vaccines are used, Engerix-B and Recombivax, as well as Twinrix (a hepatitis A and hepatitis B combination vaccine).
The CDC recommends that children receive the first dose of the hepatitis B vaccination at birth.

Sources
CDC, “Hepatitis B,” cdc.gov, Mar. 10, 2013
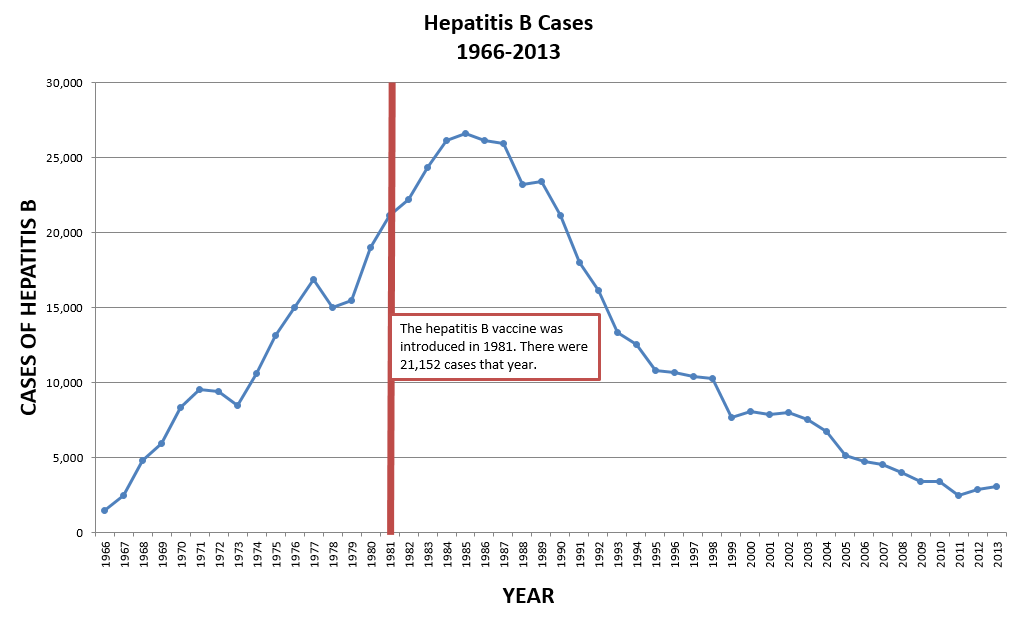
CDC, “Reported Cases and Deaths from Vaccine Preventable Diseases, United States, 1950-2013,” cdc.gov, Sep. 2014
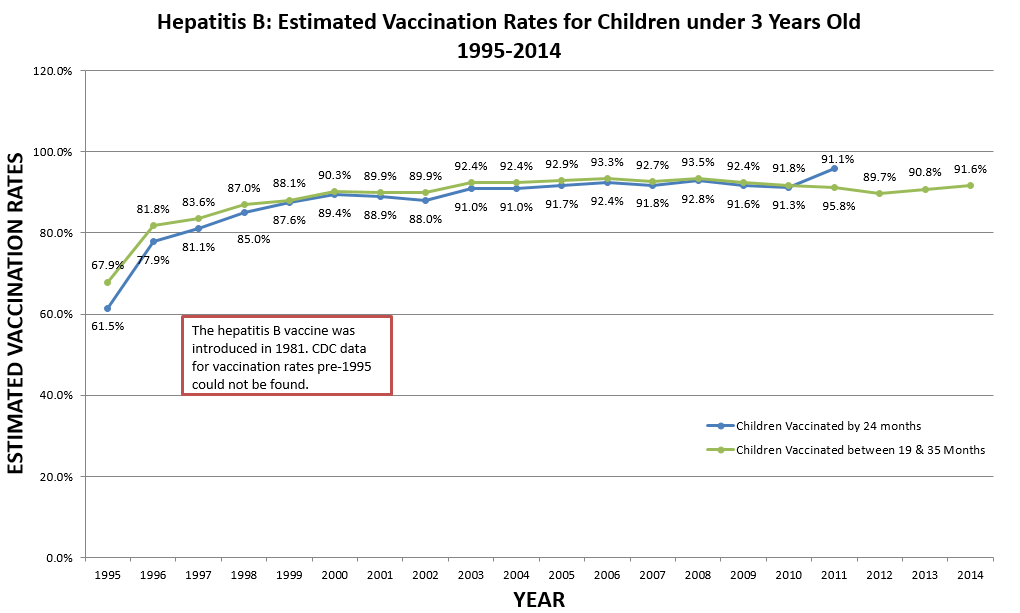
CDC, “U.S. Vaccination Coverage Reported via NIS,” cdc.gov, Mar. 11, 2014
College of Physicians of Philadelphia, “The History of Vaccines: Timelines, Diseases and Vaccines,” historyofvaccines.org (accessed June 25, 2014)
Hepatitis B Foundation, “Hepatitis B Vaccine History,” hepb.org, Oct. 21, 2009
| Measles Outbreak |
|---|
| Fighting Measles amid the COVID-19 Pandemic |
| US May Lose Measles Elimination Status Due to Ongoing Outbreaks |
| States Rethinking Childhood Vaccine Mandates during Measles Outbreak |


